Providing global access to
quality medical care
You want to help people to improve their lives by distributing and providing essential medicines, medical consumables and hospital equipment to those in need.
We at Imres have the knowledge, experience, passion and the skills to help you achieve that.

Your specific needs and particular problems are our challenges. We commit ourselves to create a turnkey, customised solution for you. Our experience in managing healthcare projects enables you to focus on your health vision.

With our extensive range of more than 4.000 products in stock we offer you a one-stop-shop solution for all your medical needs. From a wide range of medical kits and generic medicines to medical consumables and hospital equipment.

Counterfeit medicines and medical products is a growing worldwide concern. We do not take chances when it comes to the quality of the products that we supply, or to safeguarding the health of patients.

The best medicine is ineffective if it cannot get to where it is needed, when it is needed. Recognising this, Imres has made logistics an integral part of the services we offer. Our logistic solutions are designed for speed and efficiency.
Request your product guide today
This content is not visible because of your cookie settings.
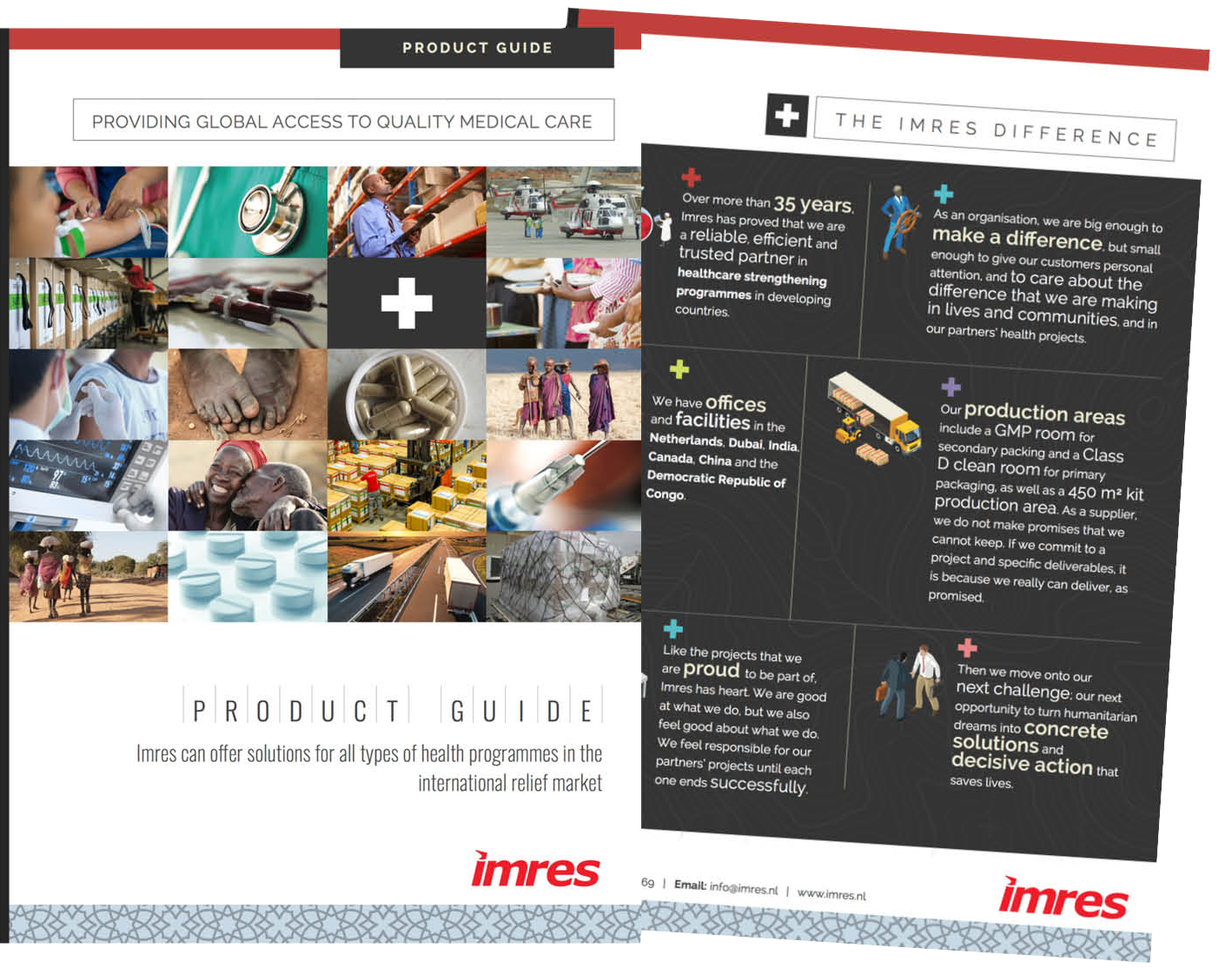
The imres difference
{{cookies.message.video}}

In a world where millions of people still do not have access to even the most basic form of healthcare, Imres strives to make a difference.
How? By supplying high quality and affordably priced pharmaceuticals, medical consumables, hospital equipment and medical kits. We are making a global impact on healthcare in the developing world by providing high quality and cost-effective medical solutions for our customers and partners.
We approach business differently, building partnerships that are founded on trust and a sense of shared responsibility. We are as invested as you are in the success of your health projects. The world’s leading aid agencies, non-governmental organisations (NGOs) and charities, as well as governmental and private organisations, choose to partner with Imres because we always deliver what we promise.
facts & figures

100 + dedicated multilingual employees globally
Offices and facilities in the Netherlands, India, China and Dubai

24/7 stock availability for immediate shipment
Widest product range 4,000 + items on stock

What our customers say
"Sourcing and delivering quality medicines in a timely manner is a serious business requiring commitment and dependability, which are qualities we have always found in Imres."
" We partner with Imres for many years now. They have proven to be a reliable, flexible and solution oriented partner who have good quality products and are willing to go the extra mile. "
8 reasons
1. You want the best quality for an affordable price, we have the expertise to source affordably priced products for your health projects
2. The outcome of your health projects depends, amongst other things, on the quality of your supplier. We have stringent QA and QC systems in place. We have all the relevant certifications and maintain the highest service levels
3. You prefer to have just one supplier for your medical and pharmaceutical supplies. With more than 4,000 product in stock we can offer you a one-stop-shop solution
4. Your health projects are often challenging. We love to develop creative and flexible solutions. Our organisation is fully equipped to help you successfully realize your projects
5. You want a partner that understands your challenges. With 40 years’ experience and being one of the largest international medical wholesalers we understand your challenges

6. You need to respond immediately when a major emergency occurs. We know that delays can cost lives, so our emergency solutions are designed for a 24/7 response
7. You feel responsible for the outcome of your health projects. We commit ourselves to support you in every way to ensure on time delivery of medical supplies
8. Communication is a critical success factor in your health project. At Imres you have a single point of contact to manage your project from start to finish

Stay informed of news & promotions
This content is not visible because of your cookie settings.
Larserpoortweg 26
8218 NK Lelystad
Netherlands
+31 (0)320-296969
info@imres.nl
+31 (0)320-296929